A global network of professional problem-solvers working with Entrepreneurs, Enterprise, Governments, NGOs, Communities, and Funders who focus on developing viable and actionable solutions to protect the health of the planet and its people to allow prosperity for all.
With an extensive background in population health, data science, and global supply chains, The New Bureau was uniquely prepared for COVID-19 rapid response programs.
COVID-19 RAPID RESPONSE:
THE SPARROW
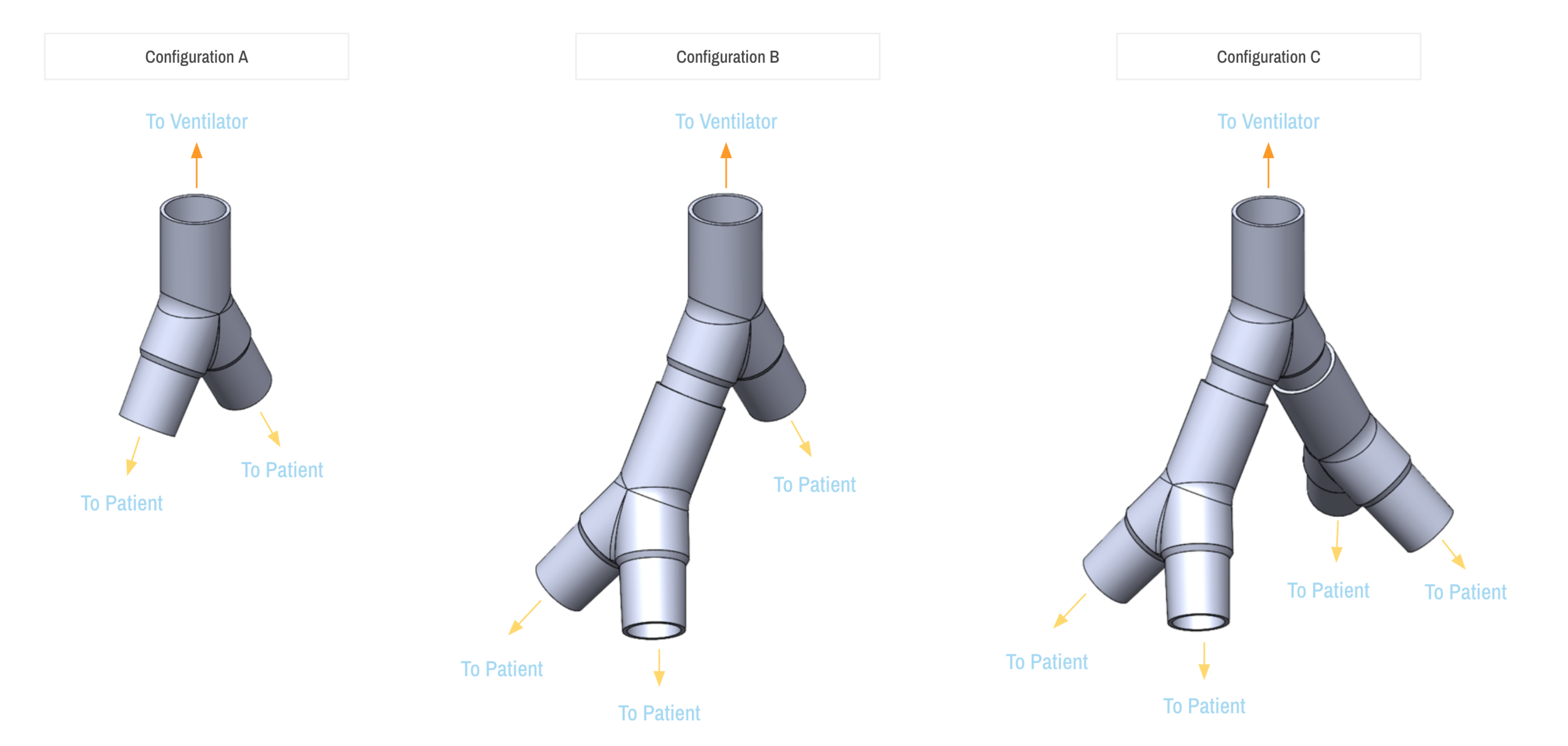
SPARROW is a new device that allows one ventilator to support up to two patients
With FDA EUA201679
In late 2019 The New Bureau identified a pulmonary disease outbreak which would later be identified as SARS-CoV-2 (COVID-19) emerging in Wuhan, China using its C8 PlanetScan Global Intelligence platform.
Using this platform we started modeling a global pandemic scenario and began monitoring pollution from emissions and healthcare systems in China to understand the scale of the outbreak and response.
We could see the manufacturing supply chain shutting down due to a reduction of emissions (N02, Me4, C02) over the locations of the Chinese manufacturing sectors that we monitor from the EAS SENTINEL-5 Environmental Satellite. We also monitor global shipping activity from a private satellite network to anticipate port restrictions and lockdowns.
With this intelligence we made a series of predictions and prepared a predictive supply chain and began sourcing PPE and other critical medical supplies for Governors, Mayors and Counties in the U.S.
Using our predictive demand models and a known lack of ventilators in existing healthcare systems, coupled with intelligence that the Chinese and EU healthcare system was failing during Wave 1, we designed and developed the SPARROW an FDA EUA co-ventilation device to rapidly prepare for a global outbreak with multiple waves of infections.
As COVID-19 results in pulmonary and cardiovascular distress, a special situation of emergency use of a product that did not yet exist was needed to improve the clinical flow and options available to the healthcare workers on the front lines. Our goal is to help healthcare facilities who only have a number of limited ventilator supplies and specialized respiratory technicians manage the stress and clinical flow of a crisis situation.
Our goal is to help healthcare facilities around the world respond to the COVID-19 pandemic.
THE SPARROW DEVICE
FDA EUA201679:
Manufacture: The New Bureau
Date of Authorization: 06/16/2020
Authorized Ventilator Tubing Connectors
Device Model: SPARROW The Single Path Airway Rapid Retrofit Optional Workflow ("SPARROW")
COVID-19 Co-Ventilator Assist Device Dual Patient Circuit Connector
FDA EUA201679
Sparrow is a new device that allows one mechanical ventilator to support up to two patients during times of acute crisis.
It has been authorized for emergency use in the U.S. during the COVID-19 public health emergency (EUA).
SPARROW has not been cleared or approved by FDA for use outside of the COVID-19 public health emergency.
The Single Path Airway Rapid Retrofit Optional Workflow ("SPARROW")
COVID-19 Co-Ventilator Assist Device Dual Patient Circuit Connector
FDA EUA201679
Intended Use The Sparrow COVID-19 Co-Ventilator Assist Device is a ventilator circuit splitter used to adapt a single mechanical ventilator for use by more than one patient when individual ventilators are not available. It is not intended to be used when the supply of available ventilators is sufficient to provide individual mechanical ventilation.
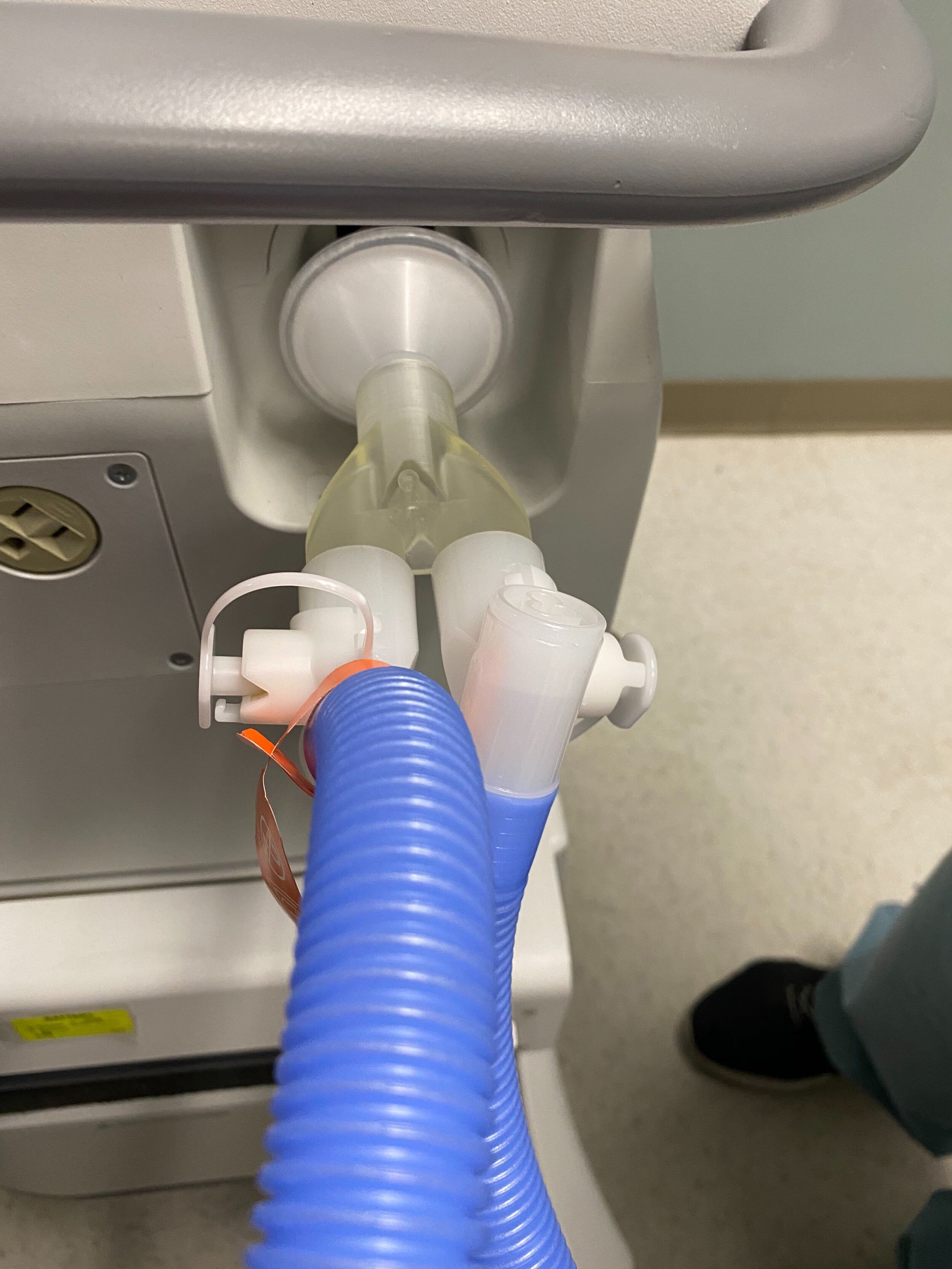
SPARROW with 2 Bio Filters
SPARROW with 2 Patient Circuits Connected
Design and Testing Process
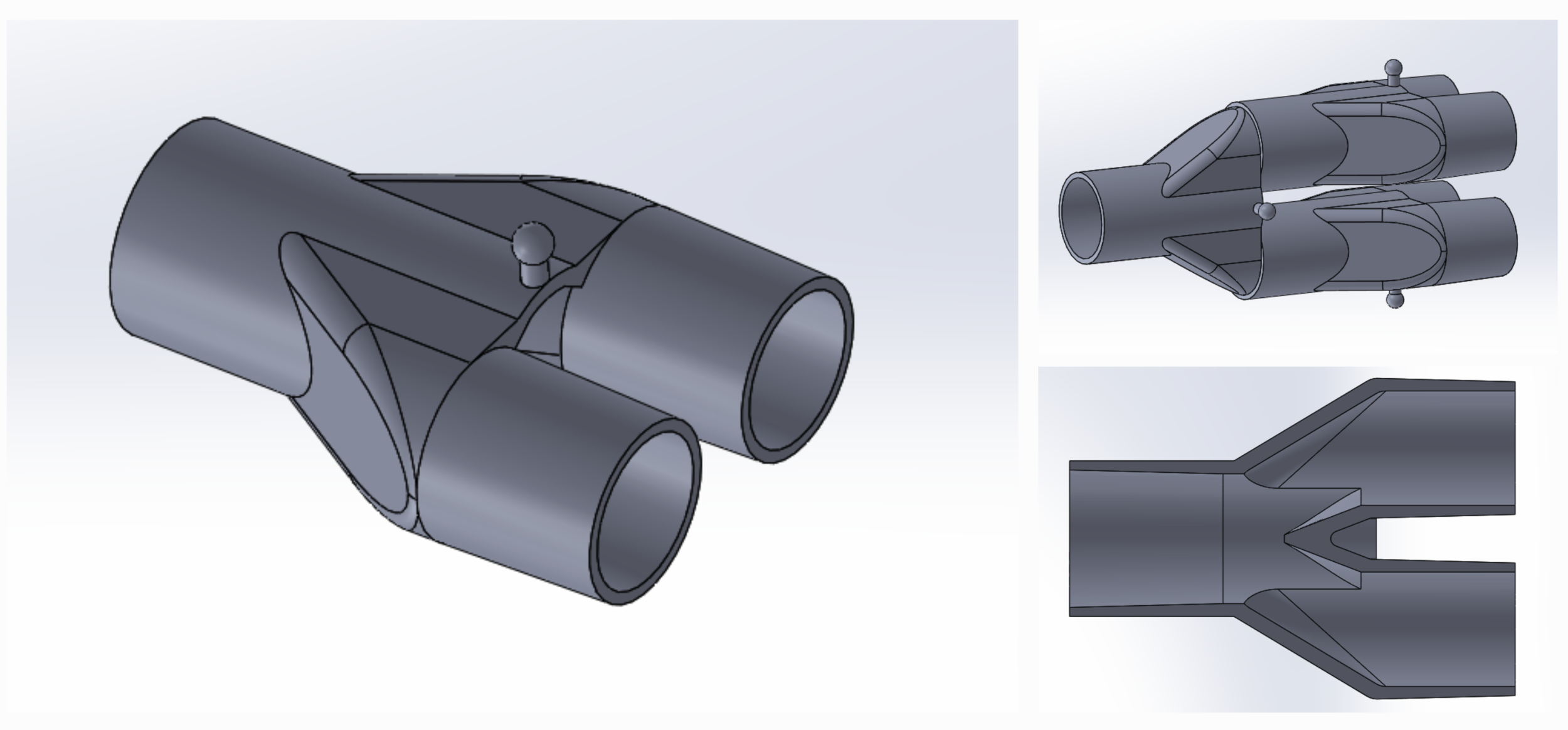
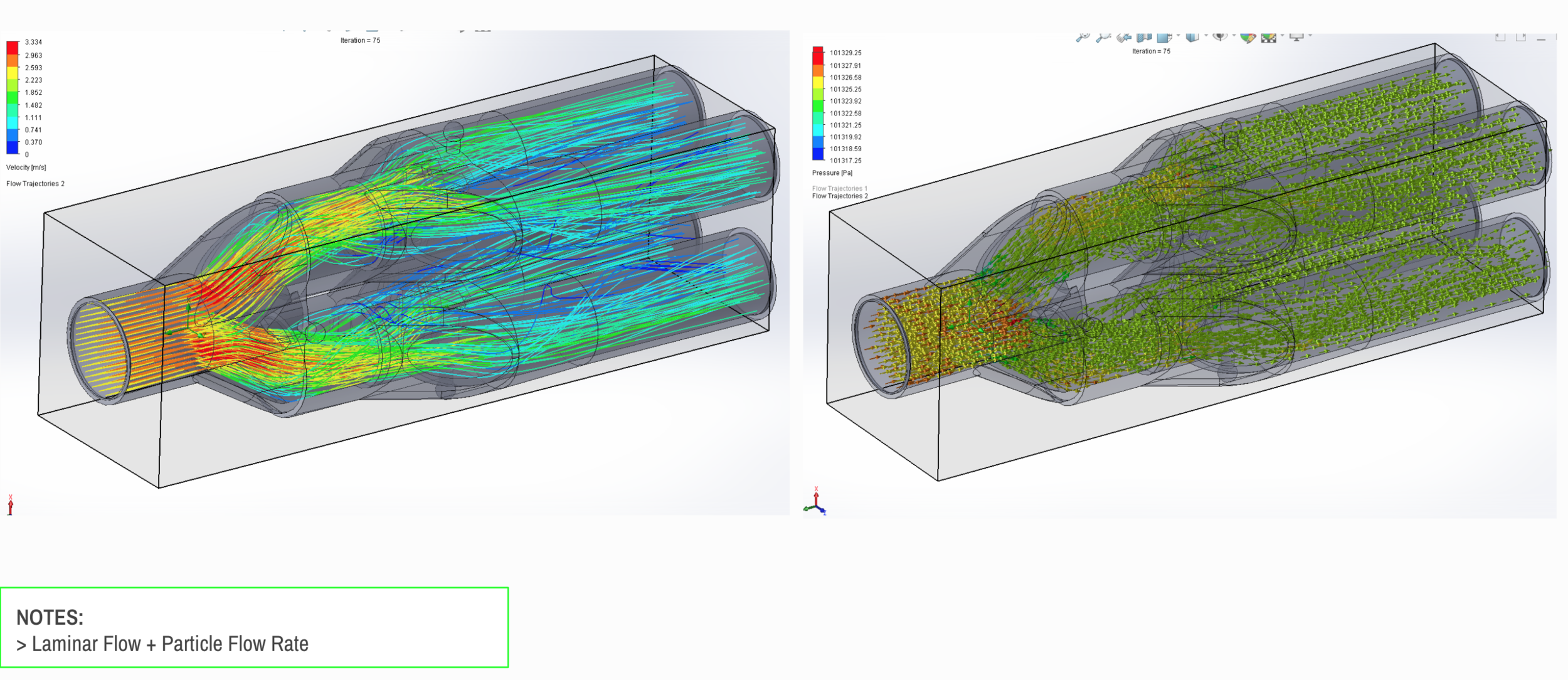

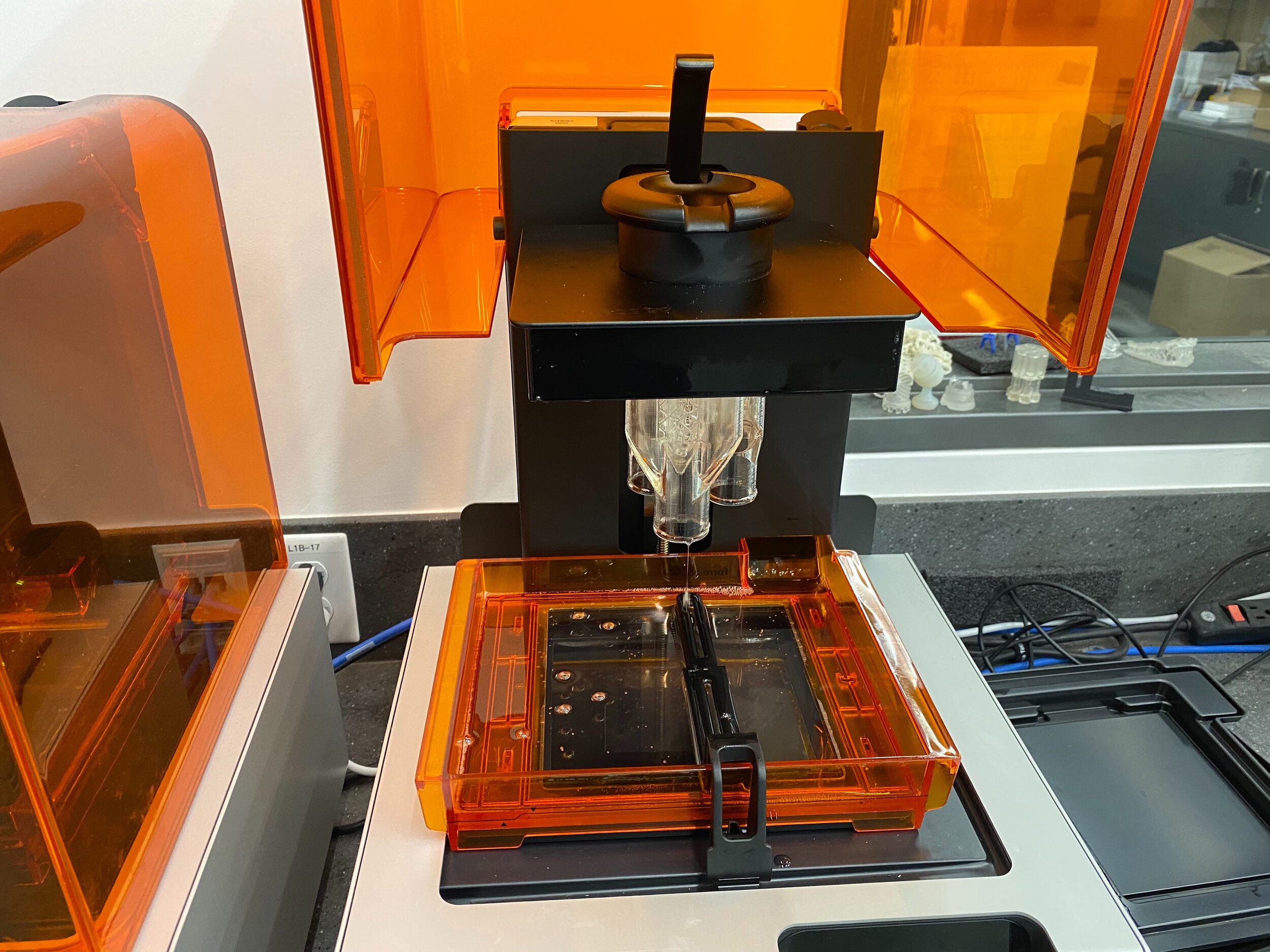






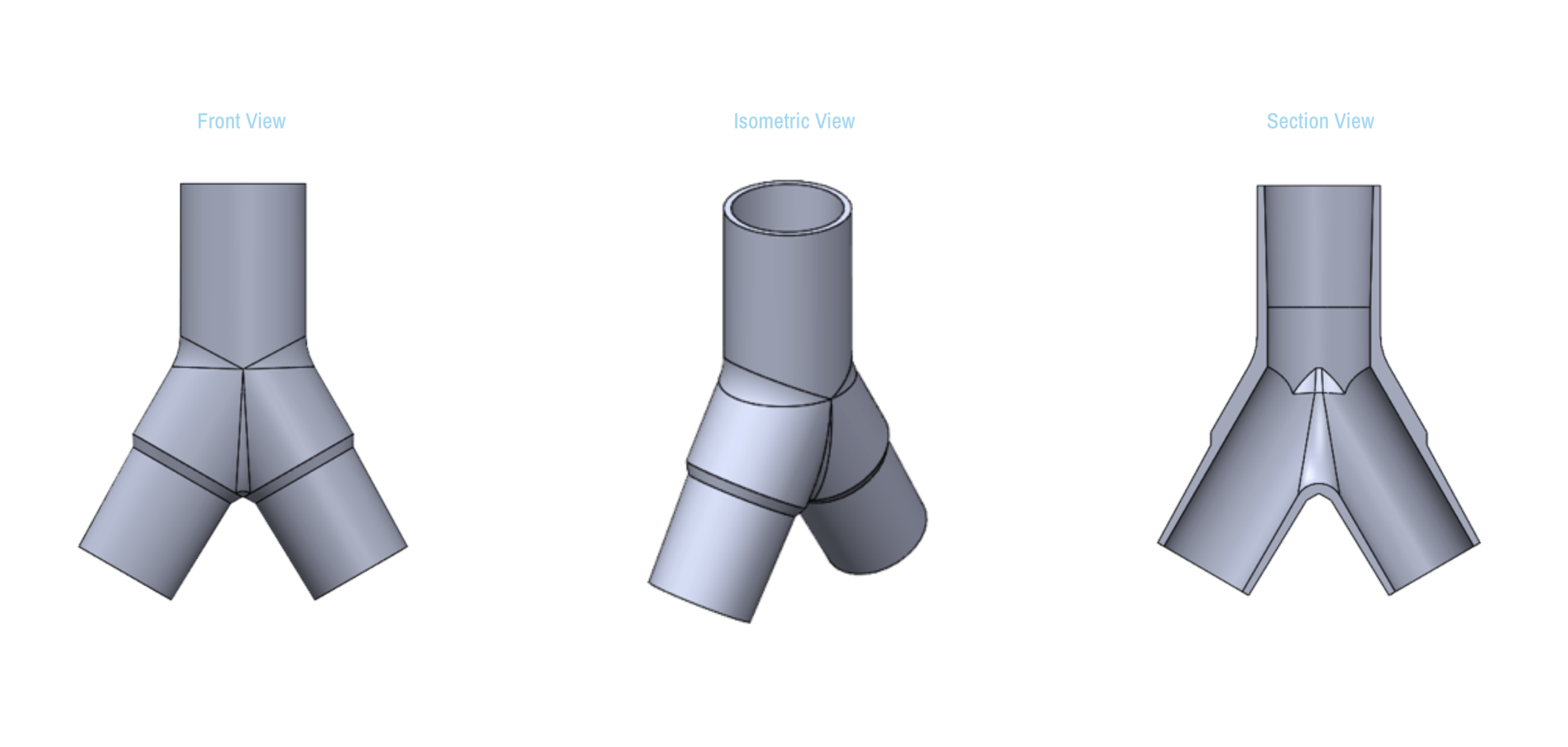
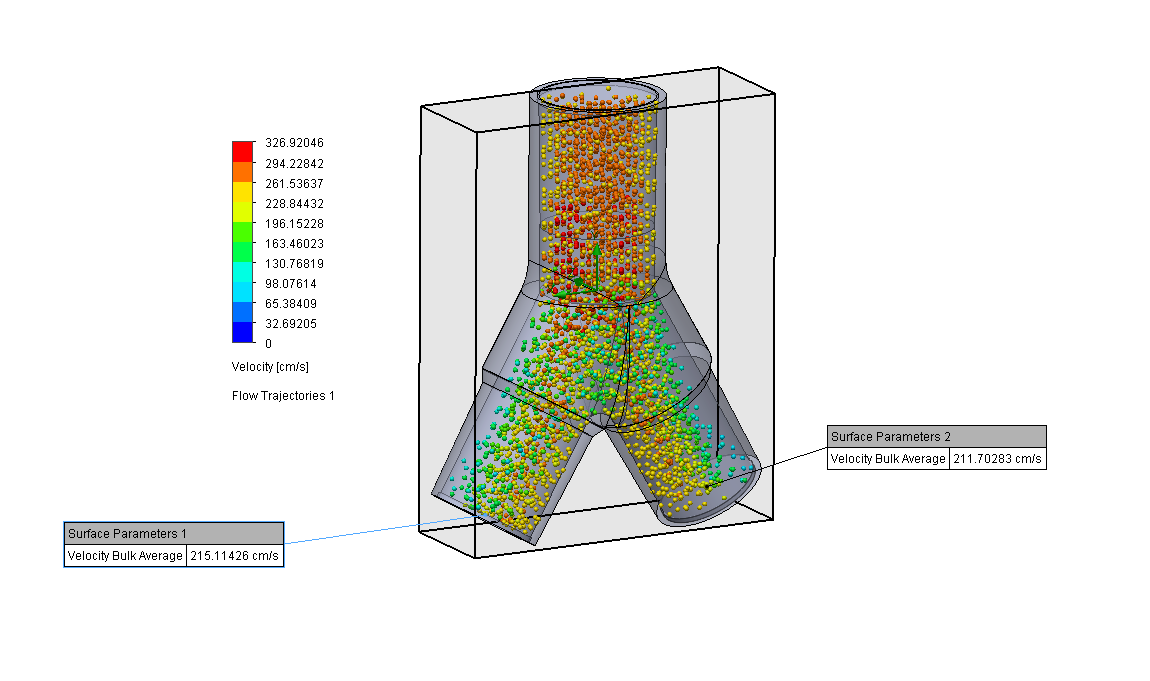
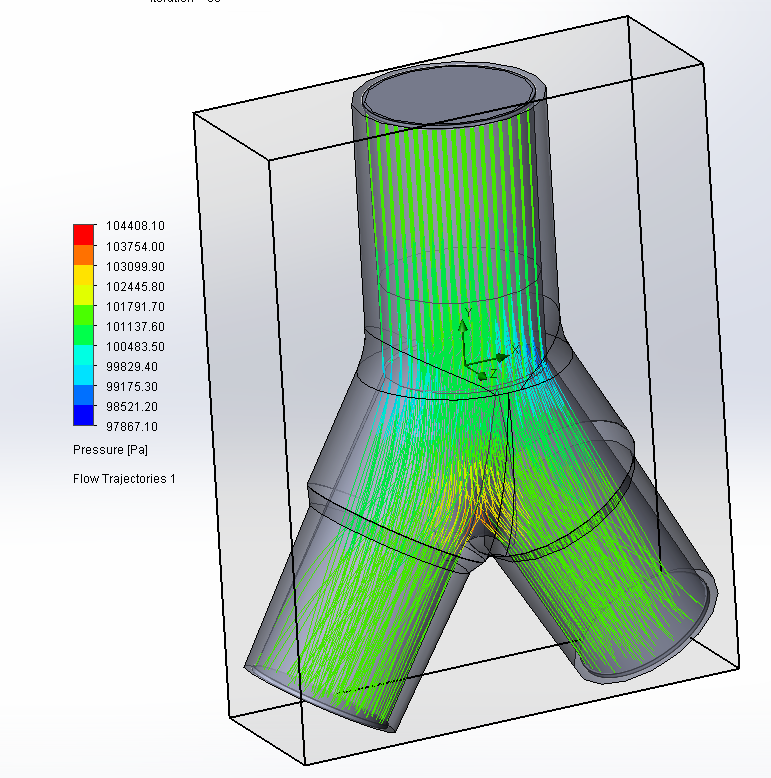
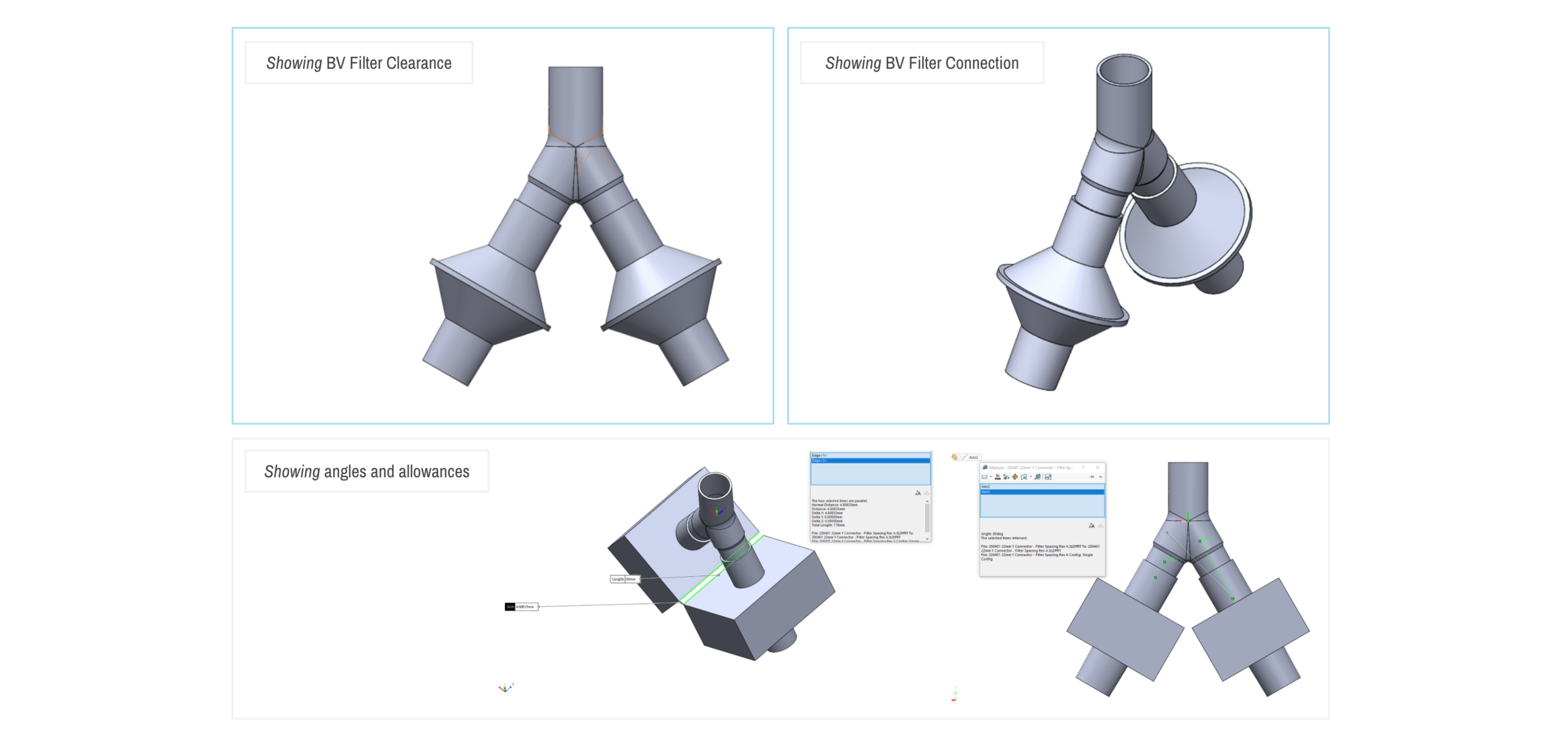
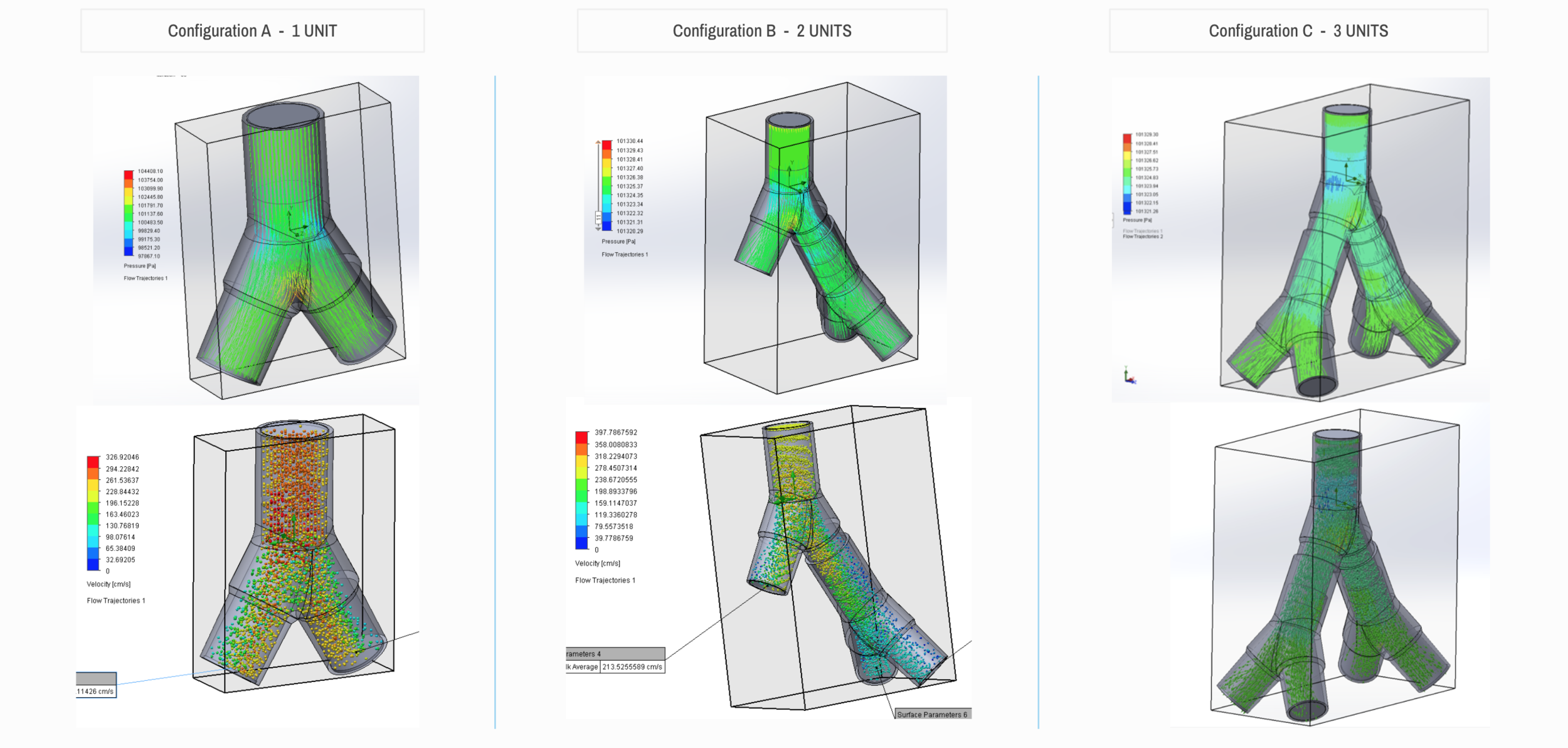
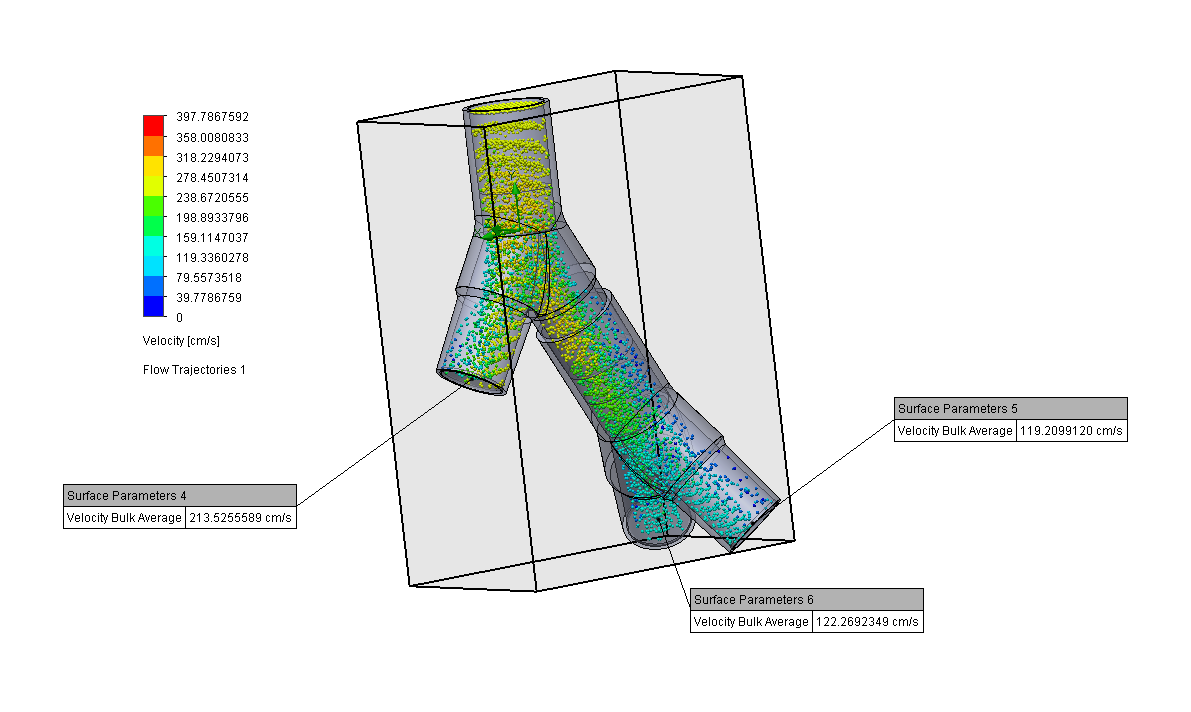
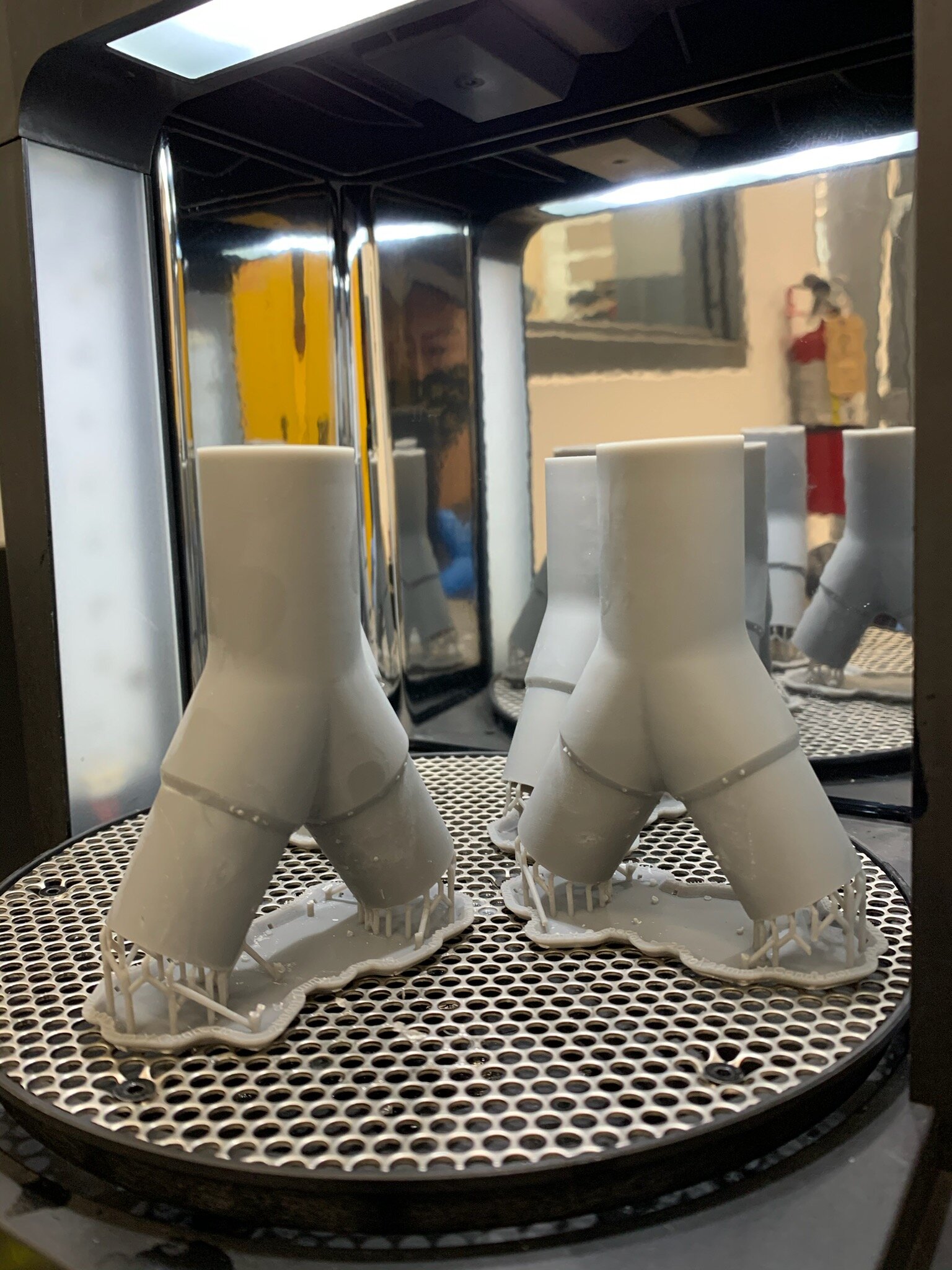
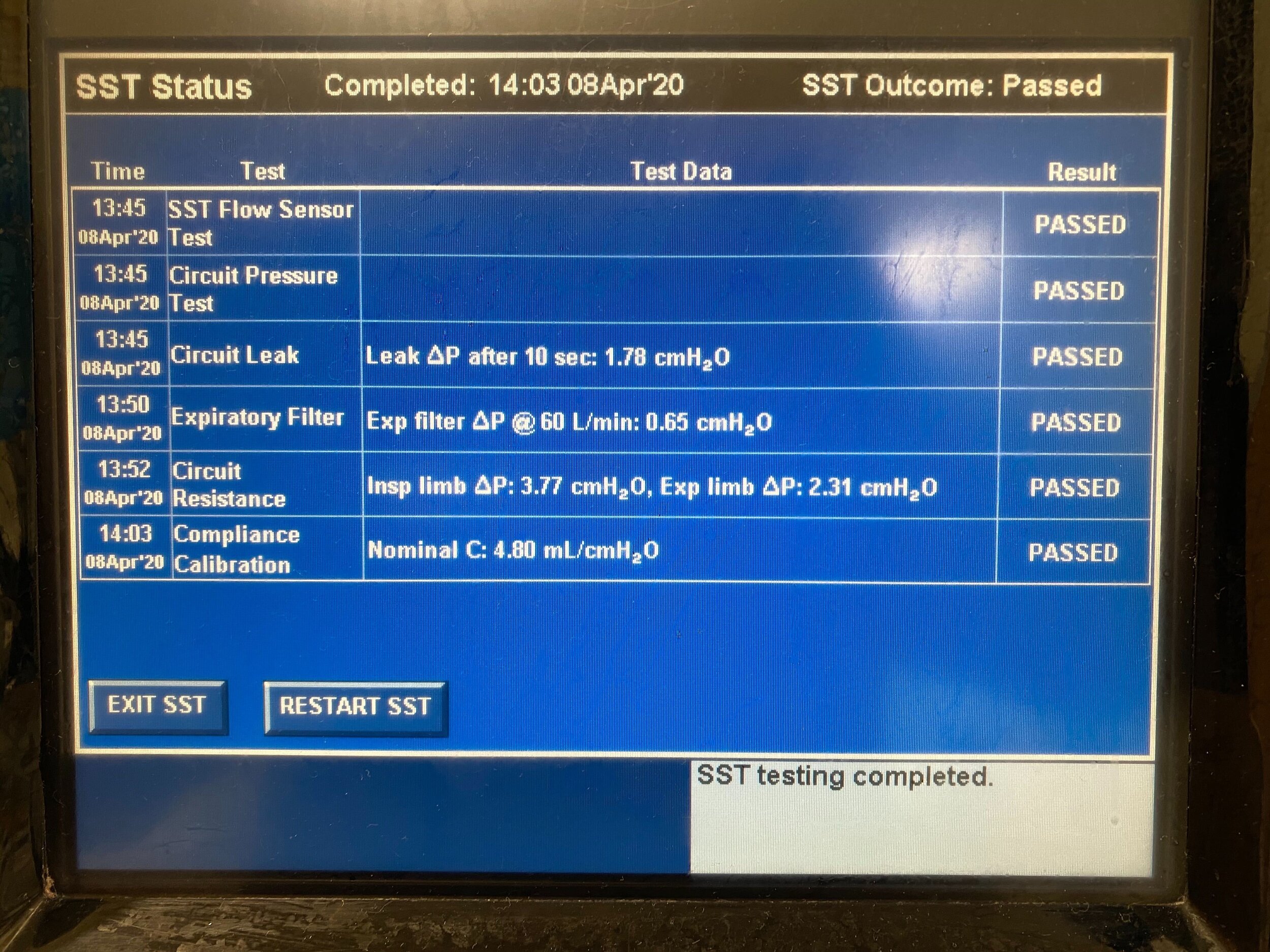
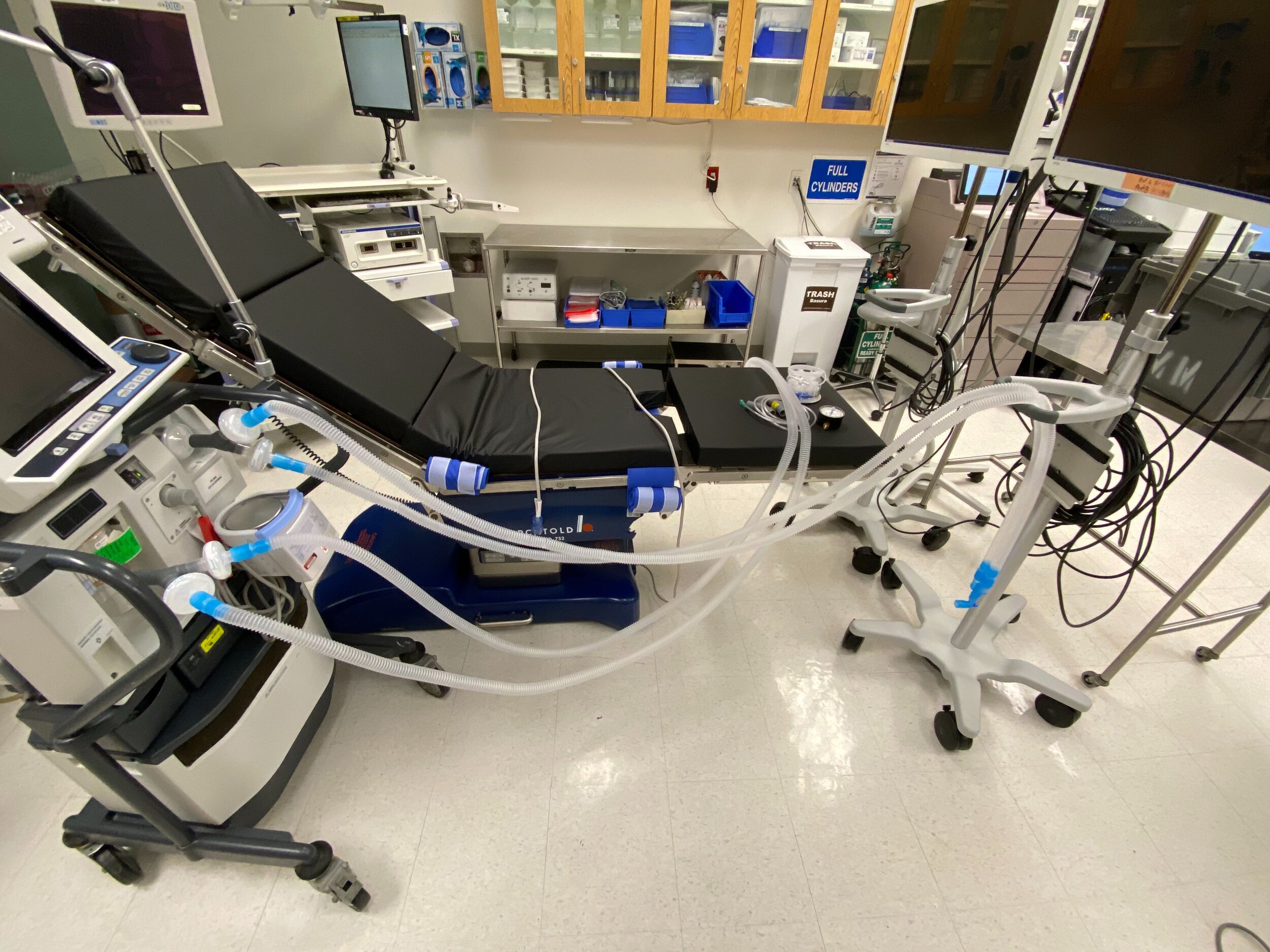
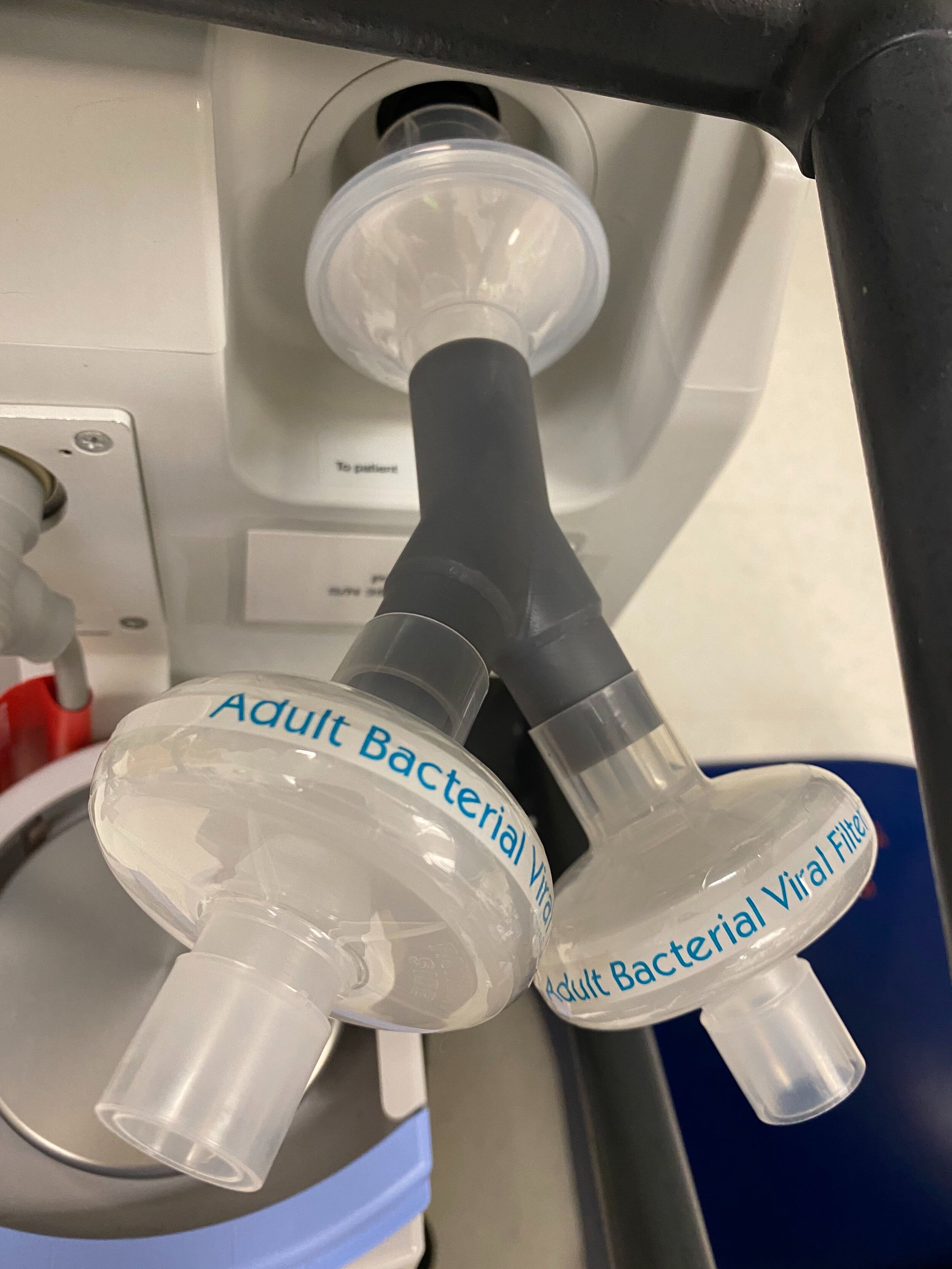
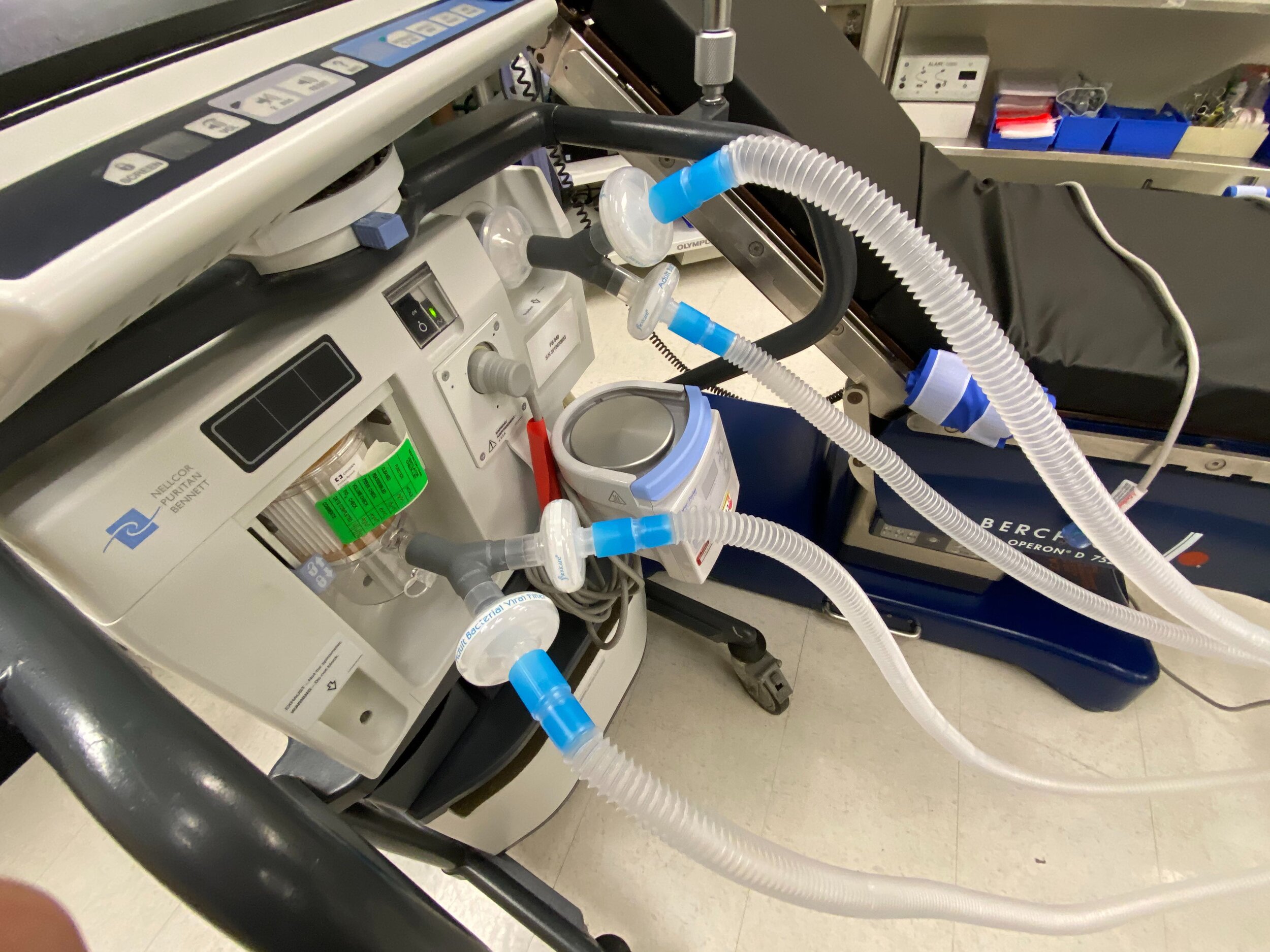






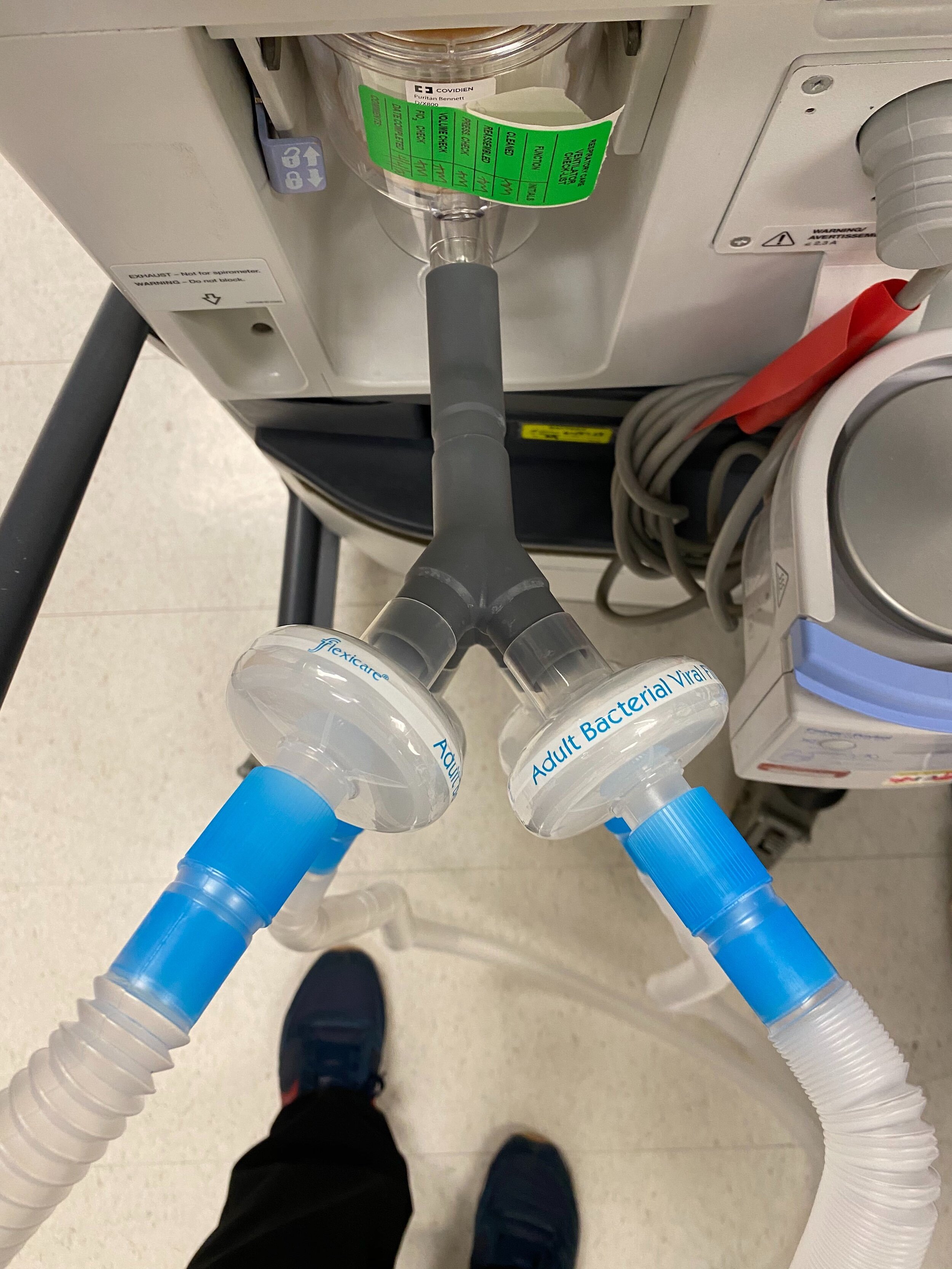
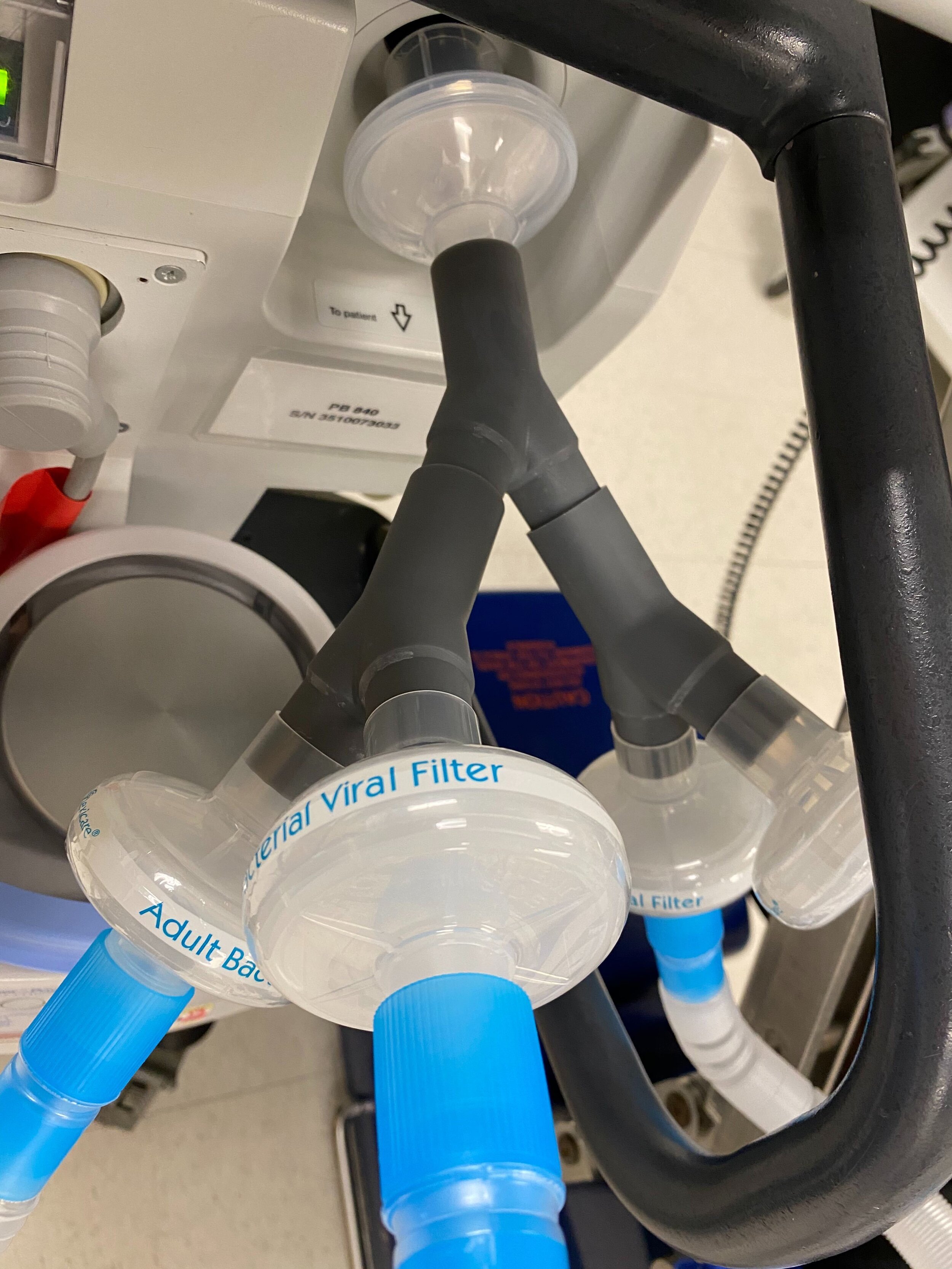

Our team developed the SPARROW (Single Path Airway Rapid Retrofit Optional Workflow) Co-Ventilator Assist Device collaborating with Tessy Plastics and the USC Pulmonary team on developing a co-ventilation protocol and clinical testing system that can be rapidly manufactured and deployed in critical areas of COVID-19.
By partnering with Tessy Plastics, a medical grade plastic contract manufacturer in Central, NY at the inception, we rapidly designed and engineered the product with a DFM process that can be ready for rapid tooling.
We worked with the USC clinical team to develop the protocol and preclinical testing, and have received a FDA COVID-19 Emergency Use Authorization (FDA EUA201679).
As we were preparing for regulatory review in parallel to the clinical and manufacturing processes we were able to do the end to end process in under 90 days.
This device can be rapidly manufactured and deployed in critical areas of COVID-19 where the hospitalization of infected patients is overwhelming the capacity of healthcare facilities who only have a limited number of ventilator supplies and respiratory technicians.
CLINICAL TEAM & MANUFACTURING PARTNER
We worked with a team at USC to develop the clinical flow, testing protocol, usage procedures, labeling, and for the FDA COVID-19 EUA.
KECK HOSPITAL OF USC
USC School of Pharmacy
CLINICAL TESTING
Daniel Stemen BS, RCP, RRT-ACCS, ECMOS - Manager of Respiratory Care and Interventional Pulmonary Services
George Sarkissian, MS, RCP-RRT - Administrative Director
Susan Bain, DRSc - Assistant Professor, Department of Regulatory and Quality Sciences, USC School of Pharmacy
TESSY PLASTICS
MANUFACTURING & ENGINEERING PARTNER
DOUGHERTY CONSULTING
FDA REGULATORY STRATEGY & RAPID EUA SUBMISSION PROCESS
We have partnered with Tessy Plastics, a medical grade plastic contract manufacturer with factories in Central NY, U.S. and Shanghai, China. Tessy has prepared laminar flow analysis, DFM, tooling, costing, and a line capable of 3,000-5,000 units a day.
New Bureau member Ed Dougherty, Principal of dougherty.consulting, developed the FDA regulatory strategy and rapid EUA Submission process for the SPARROW. His group develops global regulatory, reimbursement, and compliance expertise for our design and development team. For over 25 years, the group has worked within the U.S. as well as in the E.U., Israel, and the U.K. to prepare for U.S. launches.
SPARROW FDA - EUA Pre Submission:
M E M O R A N D U M
Date: April 9, 2020
To: EUA Review Team for Ventilators, Connectors, and Accessories Center for Devices and Radiological Health U.S. Food and Drug Administration
From: Peter E. Raymond
Re: Request for Emergency Use Authorization for the Sparrow COVID-19 Co-Ventilator Assist Device: Pre-Submission
On behalf of The New Bureau, an innovation incubator and global network of experts focused on healthcare, climate change, education, and Smart Cities, I respectfully submit the attached preliminary information to initiate a request for Emergency Use Authorization (“EUA”) pursuant to Section 564(b) of the Federal Food, Drug, and Cosmetic Act, 21 USC § 360bbb-3, for the Sparrow COVID-19 Co-Ventilator Assist Device, an open-label, modular ventilator circuit adapter intended for use to co-ventilate up to three patients on a single, continuous use ventilator in a healthcare facility. The product is not currently marketed in the US or in any other jurisdiction.
I am a population health and technology professional and have helped develop innovative and lifesaving solutions for global healthcare organizations and health systems, including nonprofit foundations, health insurance providers, and biopharmaceutical and medical device manufacturers for more than 15 years.
In January 2020, I watched as China's healthcare providers were forced to deal with an increasingly critical deficit of ventilators and respiratory support systems by reverting to “on-the-spot” solutions, including cut up ventilator circuit tubing, garden hoses, plumbing supplies, and even saran wrap and duct tape to co-ventilate multiple patients from one ventilator. In response to this troubling observation, we started to design and test a more reliable solution that could be rapidly tooled and distributed. We cannot allow our own healthcare workers in the US to be put in a similar position to keep our citizens alive.
We have prepared this submission to offer another alternative to U.S. healthcare providers who may be forced to make the difficult choice to co-ventilate their patients in an emergency.
Our proposed product has been rigorously developed and tested to allow providers to co-ventilate COVID-19 patients as safely and effectively as possible. We have taken into account the clinical workflow of an emergency and critical care provider to help facilitate crisis management by reducing the need to improvise solutions and the stress this causes clinicians, patients, and their families by offering a device that can reduce technical limitations that might otherwise complicate the difficult choice to co-ventilate.
This document presents preliminary information that we are prepared to augment in coordination with FDA to demonstrate that the Sparrow COVID-19 Co-Ventilator Assist Device meets FDA criteria for safety, performance and labeling as set forth in Section II and Appendix B of FDA’s Public Health Emergency Guidance: Enforcement Policy for Ventilators and Accessories and Other Respiratory Devices During the Coronavirus Disease 2019 (COVID-19). It is our intention to make the design documentation and other technical information available to any entity that wishes to manufacture and distribute the product for use during a public health emergency in any jurisdiction.
Please contact me directly at your convenience. My team and I would welcome the opportunity to engage FDA staff in discussion to identify any specific additional documentation or materials that may be required to satisfy criteria for Emergency Use Authorization.
We are prepared to initiate manufacturing of the product immediately and are developing a distribution channel to deliver the product quickly to states, hospitals, health systems and other entities to help address the acute need for ventilator capacity caused by the COVID-19 pandemic.
Regards,
Peter E. Raymond
CEO & Bureau Chief
The New Bureau









